The Chemistry of Insect Repellents
Written by: Sulayman Mehboob, B.Sc., M.Sc. - Microbiologist
Abstract
There are many infectious diseases today which are arthropod-borne. Insect repellents are important topical barriers providing the protection from insect-spread infectious diseases. There are two types of insect repellents; chemical derived, and plant derived repellents. The effective application of insect is by chemical repellents like N-diethyl-3-methylbenzamide which is also known as N, N-diethyl-m-toluamide (aka DEET); picaridin (aka Icaridin); and permethrin. Other topical insect repellents like IR3535, or oil of lemon eucalyptus (p-menthane-3, 8-diol also provide protection against different insects.
Categorization
Insect repellants are categorized into two types:
1. The Synthetic Chemicals
Some of the synthetic chemicals include DEET, picaridin, and IR3535 (Ethyl 3-(Acetylbutylaminopropanoate)
2. The Plant Derived Oils
Plant derivatives Includes oil of lemon eucalyptus (p-Menthane-3,8-diol), oil of citronella, and permethrin.
The first chemical insect repellents included the dialkyl phthalates (dibutyl and dimethyl phthalate), discovered in 1929; indalone, introduced in 1937; and Rutgers 612, introduced in 1939.1 By 1946, N, N-diethyl-3-methylbenzamide or DEET which is also called as N, N-diethyl-m-toluamide) was in use by the US Army and later on marketed to the public in 19561,2.
The first efficient insect repellents included smoke from cooking fires or burning tar; different plants and flowers hung in houses or on porches; or rubbing different plants, flowers, or herbs on the surface of skin. This included plant species like chrysanthemum, geranium and lantana.1 Many plant oils, such as citronella, clove, geranium, mint, nutmeg, pennyroyal, and soybean would also repel insects for short periods, but their high volatility limited their duration of effectiveness when burned in candles or applied topically1. DEET was introduced by the U.S. Army in 1946 to protect the US soldiers in insect-infested places. Insect repellents containing DEET have been used by the general public in the United States since 19574.
Download Printable PDF (1.95MB)
Chemical Structure: Synthetic Insect Repellents
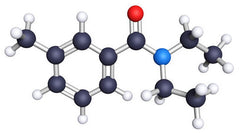
DEET Molecular Structure
Picture credit: National Pesticide Information center, USA
DEET
DEET is an insect and acarid repellent. The International Union of Pure and Applied Chemistry (IUPAC) name for DEET is N,N-diethyl-m-toluamide and is the member of the family of chemicals N,N-dialkylamide.
DEET is an insect repellent which is used in products to avoid bites from insects such as mosquitoes, biting flies or fleas and small flying insects. DEET is a liquid that has a faint odor and is colorless and does not easily dissolvable easily in water.

Icaridin Molecular Structure
Molecular Structure of Icardin
Picture credit: ACS
Icardin (Picaridin)
Icaridin,(2-(2-hydroxyethyl)-1-piperidinecarboxylic acid 1-methylpropyl ester) also known as Picaridin, or hydroxy-ethyl isobutyl piperidine carboxylate, is a cyclic amine and a member of the piperidine chemical family. Piperidines are the structural components of piperine, which is extracted from plants from the genus Piper or pepper. Icaridin has been commonly used as a topically-applied insect repellent in various countries but was officially licensed for use in the United States in 2001 and Canada in 20125.

IR3535 Molecular Structure
Atoms are represented as spheres with conventional color coding: hydrogen (white), carbon (grey), oxygen (red)
IR3535
IR3535 or 3-[N-Butyl-N-acetyl]-aminopropionic acid, ethyl ester is similar in structurally to β-alanine, which occurs naturally. The active ingredient is a liquid at room temperature.7
IR3535 is used as an insect repellent against mosquitoes, body lice, ticks and biting flies. Products which use IR3535 as the active ingredient, are applied to the exposed surface of human skin. No adverse effects to humans, nor any negative affects to environment are likely to occur from the use of 3-[N-Butyl-N-acetyl]- aminopropionic acid, ethyl ester.7
Chemical Structure: Plant Based Insect Repellents

Molecular structure of 4. p-Menthane-3,8-diol
Atoms are represented as spheres with conventional color coding: hydrogen (white), carbon (grey), oxygen (red)
p-Menthane-3,8-diol (Citridol)
p-Menthane-3,8-diol occurs naturally in the lemon eucalyptus plant. This natural oil can be extracted from the leaves of eucalyptus and twigs. To use commercially, the active ingredient is synthesized chemically. p-Menthane-3,8-diol shows a structural similar to menthol.6
p- Menthane-3,8-diol is a bio-chemical repellent derived from eucalyptus plants. This active ingredient is important in the manufacturing of the insect repellent products that are applied to human skin surface and clothing to repel insects such as mosquitoes. It can be used in two ways either as a spray and as a lotion. When the chemical is used by following to label instructions, p-Menthane-3,8-diol products are not likely to do harm to humans or the environment.6

About The Author:
Sulayman Mehboob, B.Sc., M.Sc. - Microbiologist
Sulayman has done research on various science projects and has been published in well reputed journals. Currently, he is doing research on animals and insects on various topics and some of his research projects have been completed and under review in the top journals. He loves researching plants and animals, and his aim is to continue deep study in this field.
- Brown M, Hebert AA. Insect repellents: an overview. J Am Acad of Dermatol. 1997;36:243–249.
- Katz TM, Miller JH, Hebert AA. Insect repellents: historical perspectives and new developments. J Am Acad Dermatol. 2008;58:865–871.
- Environmental Pesticide Information Center, “Deet General Fact Sheet” Date Reviewed, July 2008
- Reregistration Eligibility Decision (RED) DEET; EPA 738-R-98-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1998; pp 1-34.
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 125098, Icaridin. Retrieved April 30, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Icaridin.
- Environmental Protection Agency, “p-Menthane-3,8-diol (011550) Fact Sheet”
- Environmental Protection Agency, “3-[N-Butyl-N-acetyl]-aminopropionic acid, ethyl ester (IR3535) (113509) Fact Sheet”
- DEET (N,N-Diethyl-Meta-Toluamide). Revised Human Health Risk Assessment in Support of Registration Review; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2014.
- Picaridin: Preliminary Human Health Risk Assessment in Support of Registration Review; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2014
- -[N-Butyl-N-Acetyl]-Aminopropionic Acid, Ethyl Ester (113509) Technical Document; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Biopesticides and Pollution Prevention Division, U.S. Government Printing Office: Washington, DC, 1999.
- P-Menthane-3,8-Diol (011550) Biopesticide Registration Eligibility Document; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Biopesticides and Pollution Prevention Division, U.S. Government Printing Office: Washington, DC, 2000

